Affordable, sensitive, rapid and easy to use diagnostic tool to fight against antimicrobial resistance
Antimicrobial Resistance (AMR) occurs when bacteria, viruses, fungi and parasites change over time and no longer respond to medicines making infections harder to treat.
Each year 700,000 people globally and 33,000 people in the EU alone die from an infection caused by multidrug-resistant bacteria, making it the world’s most disastrous scourge of the future if no immediate and united action is taken today.
According to the report released by UN, international agencies and experts, drug-resistant diseases could cause 10 million deaths each year by 2050 and damage to the economy as catastrophic as the 2008-2009 global financial crisis1
It is estimated that AMR costs the EU €1.5 billion per year in healthcare costs and productivity losses2.
Antibiotic resistance develops when bacteria adapt to the presence of the antibiotic to which they are exposed. It is a natural phenomenon, but if antibiotics are not used rightly, this process would speed up. Misuse of antibiotics can occur in several areas and situations: healthcare, agriculture and the veterinary sector.
Currently, the detection of resistant bacteria requires the isolation and application of different tests, which takes between 16 and 30 hours, leading to delay in treatment and increasing the risk of mortality. Moreover, these tests are expensive, requires specific equipment and trained personal.
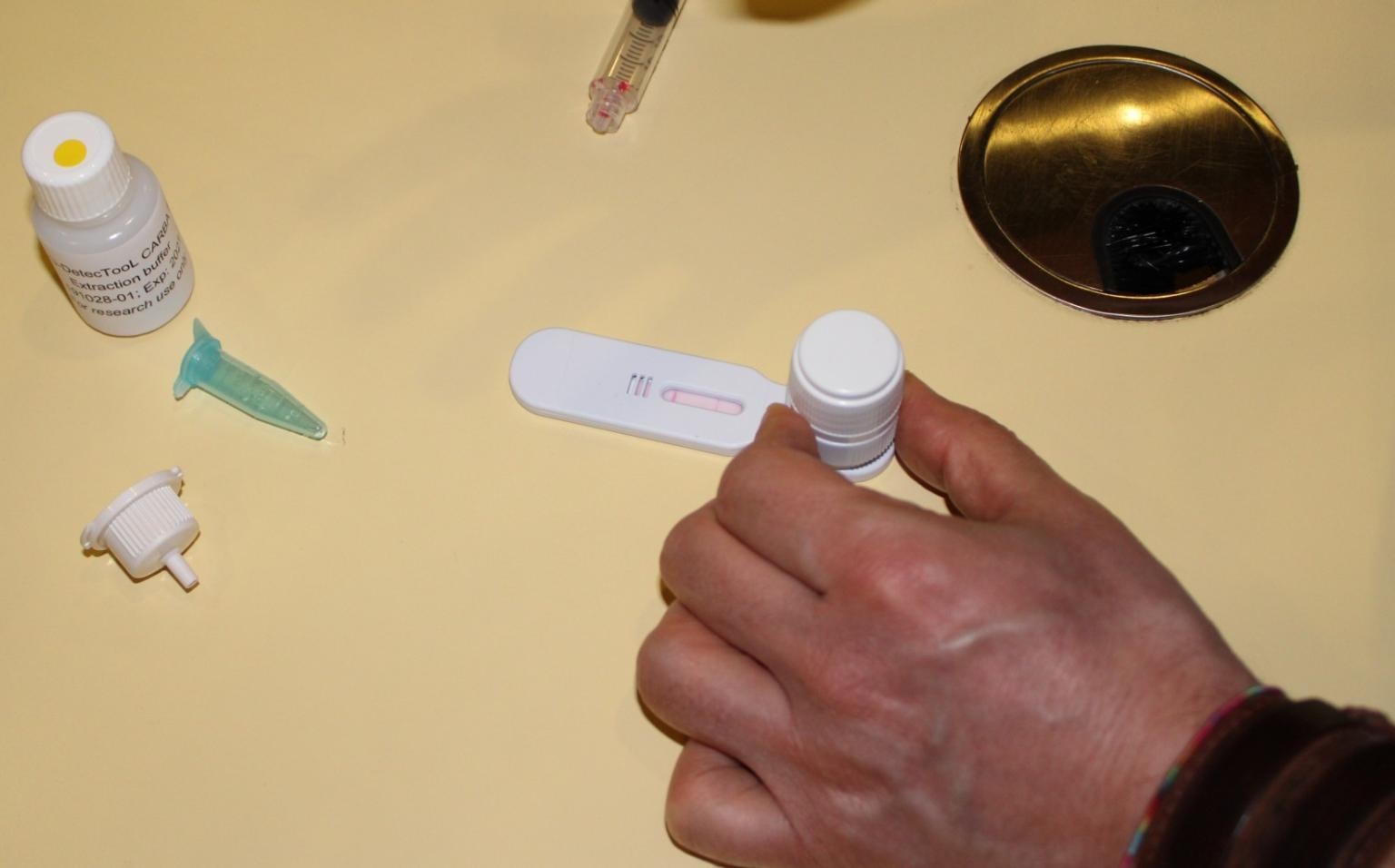
To overcome these limitations and to give patients effective treatment on time, a new detection system – the AMR DetecTool has been developed, allowing the direct and specific detection of resistant bacteria in less than 30 minutes in biological samples.
AMR DetecTool project is based on an innovation that directly detected β-lactamases in clinical samples in under 30 minutes, successfully validated during a 1-year project (BL-DetecTool) of the European Institute of Innovation and Technology – Healthin 2018 by the collaborative work of clinics, hospitals, universities, research institutes and a business partner. The objectives of the AMR DetecTool project (3-years project) are to extend the validation of the Detectool to new targets, to realize a multicentric validation (7 new hospitals from differents European countries are involved), to evaluate the cost effectiveness, to develop an AMR knowledge website and to establish a business concept.
Detectool is a new rapid detection system patented by the CEA – The French Alternative Energies and Atomic Energy Commission, a key player in research, development and innovation. Lead partners in this project are Assistance Publique – Hôpitaux de Paris (APHP), NG Biotech, University Hospital Clinic of Barcelona, IS Global, IESE Business School and Semmelweis University and the associated hospitals are Institut Català de la Salut, National and Kapodistrian University of Athens, Central Hospital of Southern Pest, CHU Amiens-Lille, Queen Mary University of London, IML of Bochum and University of Florence (UNIFI).
How does the device work?
- With this device, bacteria can be extracted directly from clinical samples such as urine, feces or blood.
- The detection is possible using a technique known as Lateral flow immunoassay.
- It relies on the use of antibodies that can recognise specific resistance markers.
- If one of the markers is present, the antibodies will bind to it, and the colour band will appear on the test.
- The second band known as the control line will also appear to indicate that the test has been run successfully.
- If no resistance marker is present in the sample, only the control line will appear.
It is easy, rapid and inexpensive to use than the currently available tests.
Milestones:
- 2018 – The BL-DetecTool project concept is developed and evaluated in three hospitals
- 2019 – The AMR Detectool project starts. The tests are industrialised and optimised
- 2020 AMR DetecTool launches the production of the first thousand tests, which will be evaluated in ten European hospitals. The project Website is launched.
- 2021 – Launch of the production of thousand new tests (new targets), which will be evaluated in ten European hospitals. Production of handheld test readers, evaluate the cost effectiveness, develop an AMR knowledge website
- 2022 – Finalization of the test validation, establish a business concept
“This project will accelerate and facilitate the marketing and implementation of an innovation resulting from research conducted at the CEA.
The main objective of this project is to save time to save lives.”
Hervé Volland, researcher, CEA
“Our range, composed of 5 products, has been developed in partnership with CEA and AP-HP”
Milovan Stankov Pugès, CEO and Founder, NG Biotech
“I think the most important change now is the replacement of the standard molecular techniques based on PCR by the rapid lateral flow in a near future”
Jonathan Lellouche – Head of Laboratory, National Institute for Antibiotic Resistance & Infection Control, Ministry of Health, Israel
“A very interesting product in that I could give a clinician advice under carbapenemases producing organism whether is present or absent in a blood culture when it becomes positive as opposed to waiting 24 hours to give them a result on that and they can direct patient care more appropriately”
Sana Tansey – Senior Medical Scientist, Irish National CPE Reference Laboratory, University Hospital Galway, Ireland
EIT Health partners involved in the project
- CEA – DectecTool is patented by CEA – a key player in research, development and innovation.
- NG Biotech – a French biotech start-up external partner in the project.
- Assistance Publique – Hôpitaux de Paris (APHP)
- University Hospital Clinic of Barcelona
- IS Global
- IESE Business School
- Semmelweis University
Associated Hospitals – Tests and reader validation
- Institut Català de la Salut
- National and Kapodistrian University of Athens
- Central Hospital of Southern Pest National Institute of Hematolgy and Infectious
- Diseases (DPC)
- Centre Hospitalier Universitaire Amiens-Picardie
- Queen Mary University of London
- Institut für Medizinische Laboratoriumsdiagnostik Bochum (IML Bochum)
- University of Florence (UNIFI)
Sources
2https://ec.europa.eu/health/antimicrobial-resistance/eu-action-on-antimicrobial-resistance_en