Ionic miniature polymer actuators for minimally invasive interventions
The University of Tartu has developed a high-performance polymer actuator technology (iEAP) that can be used to make a minimally invasive device with better steerability, for improved treatment of conditions such as chronic arterial diseases. This project will assess the feasibility of using the technology in Philips’ minimally invasive interventional devices.
Origins
Chronic arterial diseases in the brain or legs affect hundreds of millions of people worldwide. Steerability of interventional devices shortens treatment time and potentially lowers X-ray dose by improving navigation and access to small and hard to reach blood vessels, enabling better treatment of chronic vascular diseases and improving patients’ lives.
Team
The consortium is composed of two members who have complementary expertise: The University of Tartu spawned the technology and has developed it up to a maturity level where it is ready for transfer to industry. Philips is the industrial member who will assess if the technology performs adequately for implementation in the intended application(s).
The project
The project will use the iEAP innovation in a minimally invasive device to create a demonstrator model that has steerability on demand. The goal is to validate the usefulness of the new technology and assess the performance in this phantom model with intended users, to determine if the solution is suitable for further development towards market introduction.
In addition, several educational activities are planned, to highlight key aspects of technology transfer from academia to industry.
EIT Health contributes in facilitating the partnership, on one side by allowing the academic stage technology to be matured in key directions that are most relevant for industry, and on the other side by providing the framework for evaluation of the technology in a specific application case.
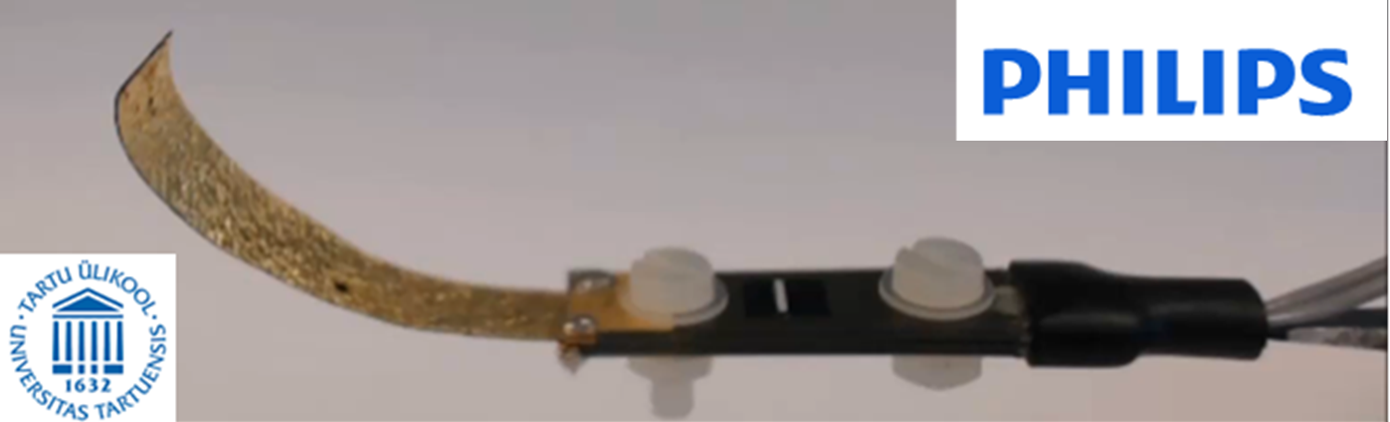
Impact
If the project validates this innovation, it can lead to introduction of a new and better a minimally invasive device for treatment of vascular conditions. The improved steerability will enable access to more branched and tortuous vessels. The innovation also offers promise for other areas where such a device could be helpful. Patients and society will benefit from improved treatment. By simplifying non-invasive procedures, the innovation makes it possible to use these procedures more widely.
Why this is an EIT Health project
This project is an excellent example of EIT Health’s effort to unite the various side of the Innovation Triangle because it brings together a development created in an academic research environment with the market knowledge of a major corporation.
The project is also in line with the EIT Health Focus Area of “Care Pathways” because it promises greatly improved care for a widespread condition.
Members

Partner classification: Business
At Philips, our purpose is to improve people’s health and well-being through meaningful innovation. We aim to improve 2.5 billion lives per year by 2030, including 400 million in underserved communities.
We see healthcare as a connected whole. Helping people to live healthily and prevent disease. Giving clinicians the tools they need to make a precision diagnosis and deliver personalized treatment. Aiding the patient's recovery at home in the community. All supported by a seamless flow of data.
As a technology company, we – and our brand licensees – innovate for people with one consistent belief: there’s always a way to make life better.
Philips Electronics Nederland B.V.
Philips Electronics Nederland B.V., Boschdijk 525, 5622 Eindhoven, Netherlands
Key Activities in Corporate Innovation
Med Tech, ICT


CLC/InnoStars: Scandinavia
Partner classification: Education, Research
Partner type: Core Partner
The University of Tartu (UT) is the oldest and largest university in Estonia and is one of the leading education institutions teaching medical and health sciences. The UT is a core partner of EIT Health in the Scandinavian co-location centre and actively collaborates with its linked third party, Tartu University Hospital, and Tartu Biotechnology Park, the Regional Innovation Scheme hub of EIT Health in Estonia. The UT has a portfolio of successful spin-offs in medtech, biotech, pharma etc., and is involved in several business incubators and student training networks. Tartu University Hospital is the largest teaching hospital in Estonia and a strong partner in research and education, providing direct access to clinical trials and patient feedback. The Estonian Biobank owned and curated by the UT is a population-based biobank, consisting of samples and rich eHealth data (from national registers) of over 200,000 gene donors. The Institute of Genomics has a strong competence in genomics, metabolomics, etc. The expertise of the UT Faculty of Medicine includes R&D competencies in innovative anticancer drug candidates, point-of-care diagnostics of infectious diseases, also nutritional and behavioural sciences. Estonia has many encrypted digital population-wide data repositories incorporated into government functions that link the nation’s various databases through end-to-end encrypted pathways (incl. digital medical records, e-prescriptions), opening up piloting opportunities for innovative healthcare solutions and new business models. The University of Tartu is in the lead of developing the Estonian healthcare system by offering its residents genome-wide genotyping that will be translated into personalised reports for use in everyday medical practice through the national e-health portal. The UT’s ambition is to develop and scale up personalised medicine and healthcare solutions in cooperation with EIT Health, national and local governments, startups and companies. The UT is interested in offering its unique competencies in personalised medicine and e-health, population-wide data repositories, using Estonia as a test site for innovative solutions, access to graduate students, acting medical professionals and patients (in Tartu University Hospital) and cooperation with the National Health Board, Health Insurance Fund and other stakeholders.
Key Activities in Corporate Innovation
Medtech, Pharma, Diagnostics, Consumer products, Nutrition, Personalised medicine
Key Activities in Social Innovation
Healthcare provision
Key Activities in Business Creation
Incubation, Technology transfer, Testing & Validation
Key Activities in Education
Entrepreneurship training, Medical faculties, Healthcare professional education/training
