18th August 2022
ProVerum is a medical device start-up that was co-founded by Dr. Ríona Ní Ghriallais and Dr. Conor Harkin in 2016,after participating on NUI Galway’s BioInnovate programme, which is supported by EIT Health. The founders wanted to help men suffering with BPH, who couldn’t get relief from medication, or didn’t want to go through the often-major surgery required to correct the problem.
The ProVerum team came up with ProVee, a ‘stent-like’ expander designed to gently ‘open-up’ the obstructed urethra without heating, piercing, cutting, or removing part of the prostate. The delivery system for the expander is thinner than the currently available BPH treatment options and the straightforward procedure is intended to be performed in a doctor’s office setting.
Setting innovation in motion
When starting out, accessing key stakeholders, adequate funding, and market insights to develop an idea is vital.
Grant funding
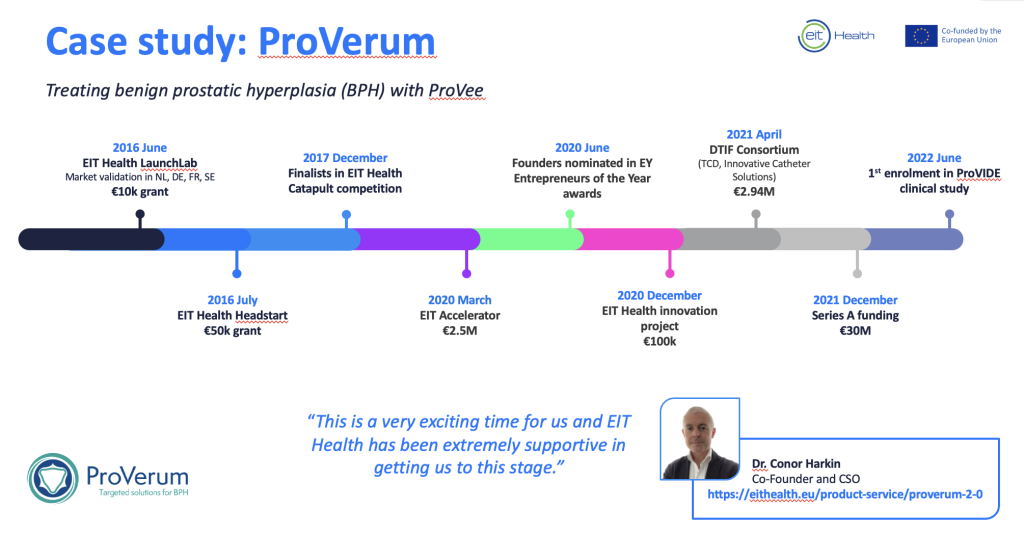
EIT Health awarded ProVerum €50,000 through the HeadStart programme in 2016. This enabled the company to spin out from Trinity College Dublin.
Market insight
ProVerum participated on LaunchLab in 2016, where EIT Health supported the team with market validation in France, Germany, the Netherlands, and Sweden.
Exposure and introductions to key stakeholders and investors
- Pitched at the EIT Health Summit 2016 in Barcelona.
- Finalist in EIT Health’s Catapult pitching competition in 2017. Catapult showcases Europe’s most promising health start-ups to leading experts and investors.
- Invited by EIT Health, the team pitched at the Global Investor Forum 2017.
- Pitched at the EIT Health Journalists Thematic Networking Meeting in Paris 2017.
- Pitched at the EIT Health Ireland-UK Start-up Showcase 2020.
Facilitating connections to world-class health experts
- International consortium: Won funding of €100K by EIT Health as part of an innovation project in 2019/2020. Joined international clinical, R&D, and business experts from Germany and Spain to optimise BPH treatment.
- User validation: in 2019, ProVerum carried out a First in Man safety and feasibility study in Australia, which reported promising outcomes with respect to symptom relief, urinary flow rates, post procedure catheterisation rates and preservation of sexual function. During the 24-month period post implantation, there were no unanticipated device related complications and no reported device or procedural related serious adverse events in any subject.
“ProVerum is helping to improve the care pathway for patients and health systems alike. Their innovation will help deliver less-invasive treatment for BPH that promises to improve outcomes for patients and reduce complications related to surgery.” – Lucía Novoa, Innovation Lead for EIT Health Ireland-UK.
Ready for investment
- Awarded €2.5M in funding by the EIC’s Accelerator project in 2020.
- €2.94M from the Irish government Disruptive Technologies Innovation Fund in 2021.
- Closed a €30M Series A investment in December 2021.
With medical device expert Paul Bateman now at the helm as CEO, supported by an experienced management team, the company has grown to 23 employees. Device patent applications have been filed in Europe, US, Japan, and China.
ProVerum is currently overseeing a 225 patient multi-centre clinical trial to evaluate the safety and effectiveness of the ProVee expander in patients with lower urinary tract symptoms secondary to BPH.
“This is a very exciting time for ProVerum and EIT Health has been extremely supportive in getting us to this stage. We are looking forward to bringing our patient-friendly solution to market so we can offer BPH sufferers a safe and effective treatment for BPH that can be delivered in the doctor’s office.” – Dr. Conor Harkin, Co-Founder and CSO, ProVerum.
Read the case study in full to learn more about how ProVerum raised €40M investment in five years.
Be part of Europe’s largest health innovation network
If you are an innovator at a high-potential start-up and would like to find out what EIT Health programmes are available to you, contact the team at EIT Health Ireland-UK today clc.ireland-uk@eithealth.eu
Yale Medicine, Enlarged prostate (Benign Prostatic Hyperplasia). Available at: https://www.yalemedicine.org/conditions/enlarged-prostate-benign-prostatic-hyperplasia-bph
Markets and Markets, Prostate Market by Disease Indication, 2021. Available at: https://www.marketsandmarkets.com/Market-Reports/prostate-health-market-107055093.html
Europe's top health start-ups take centre stage: EIT Health Catapult winners are revealed at HLTH Europe

2025 Catapult programme winners announced.
Finding Europe’s next healthtech leaders: Insights from Antoine D’Hollander

Insights from Antoine D’Hollander, Capricorn Partners.
EIT Health supports 17 promising deep tech start-ups bridge the ‘Valley of Death’

Providing start-ups with the right support.