20th August 2018
SensArs’ first product SENSY allows amputees to naturally feel their missing limbs
Meet SensArs Neuroprosthetics – one of the winners of the EIT Health Germany HeadStart Award last month and a finalist in this year’s European Health Catapult – a collaboration between EIT Health and Health Axis Europe. SensArs’ first product SENSY allows amputees to naturally feel their missing limbs. Leg amputees – not having sensory feedback from their prosthesis – cannot sense obstacles, slopes or holes, thus risk stumbles and falls continuously.
Many report phantom pain from the missing extremity and do not feel the prosthesis as part of their body (low embodiment). In many cases these factors cause abandonment of the prosthesis. Considering there are an estimated 3.18 million people in the EU with lower limb amputations and about 295 000 amputations performed annually, the market for improvement in this field is huge.
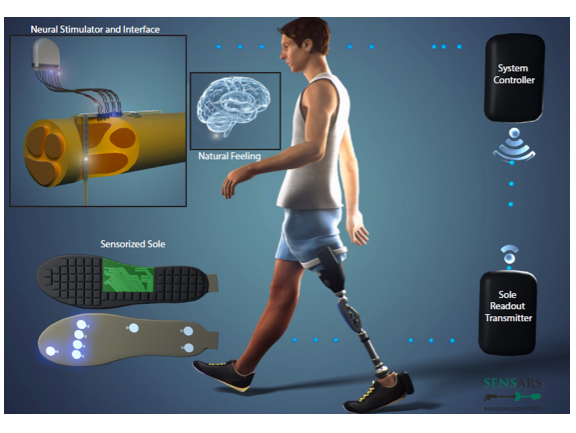
The device comprises implantable intraneural electrodes, an implantable neurostimulator, an external controller and a sensorised sole (illustrated in the image above). The neurostimulator is implanted in the limb and is wired to SensArs’ revolutionary intraneural electrodes that are inserted transversally into the peripheral nerves.
Stimulation of sensory nerves is driven wirelessly by an external controller, which transforms the readout of the sensors embedded in the sensorised sole into stimulation parameters. The sensorised sole is adaptable to the prostheses currently available in the market (e.g., Rheo Knee, C-Leg).
SensArs have received more than € 900 000 in grants (startup.ch/SensArs) which have been used to develop the first prototype of SENSY, and to test it in three leg amputees. Results convincingly show that SENSY reduces the risk of falls, increases the embodiment of the prosthesis, and diminishes phantom limb pain.
Next steps:
SensArs have defined the technical activities to reach commercialisation and a detailed analysis of the go-to-market strategy. “Perhaps the most important of these was the final specifications for the minimum viable product to be commercialised, both the hardware and software,” states Dr Petrini.
For clinical trials, the future partners were identified and contacted to perform final clinical trials in two different sites. Also, planning to collaborate with other institutions Europe-wide is ongoing for other proof of concept clinical trials to assess the feasibility of using the SENSY product for different indications.
SensArs have identified and planned for the required manufacturing practice accreditations. Evaluations were scheduled to obtain the International Organisation for Standardisation (ISO) certification and the CE mark.
A feasible preliminary industrialisation plan was established and business operations were elaborated. The team identified all the value chain actors and outlined the manufacturing and distribution infrastructure.
In total, 60 patients will participate in clinical trials planned at the Policlinico Gemelli Hospital in Rome and the Charité Clinic in Berlin. “After this milestone, we will apply for the CE mark and start commercialisation of the SENSY in Italy and Germany, ready for expansion throughout the rest of Europe,” sums up Dr Petrini.
Click on the links below to learn more:
EIT Health supports 17 promising deep tech start-ups bridge the ‘Valley of Death’

Providing start-ups with the right support.
EIT Health launches seven new members to its community

Enriching the EIT Health network with their collective expertise.
Driving personalised medicine forward

The second EP PerMed Round Table convened in Valencia and online.