Novel urine test for early detection of colorectal cancer metastases in the liver
The COLO-MET project aims to develop a novel cost-effective and patient-friendly urine test, combined with a blood test, for early detection of liver metastases related to colorectal cancer.
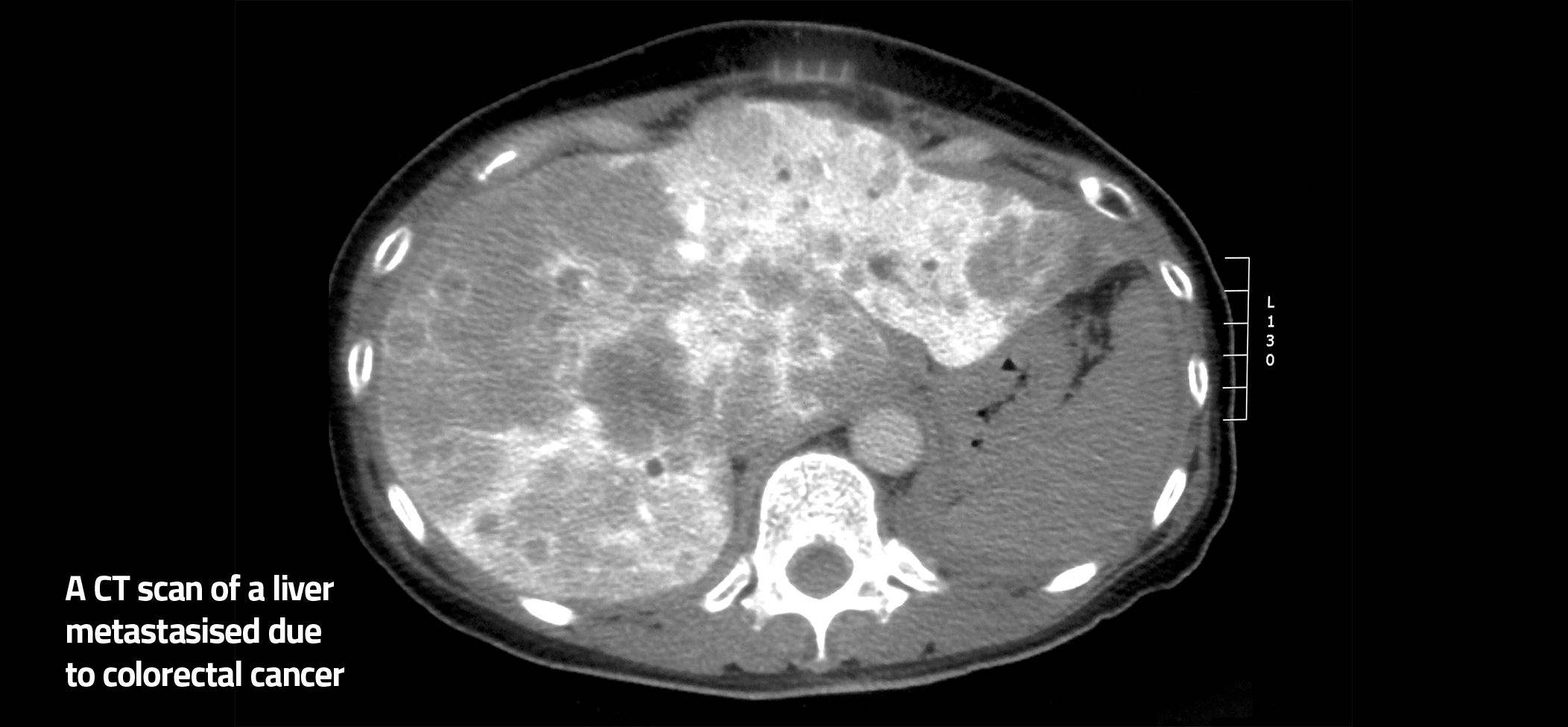
Bowel cancer is the third most common cancer, with 400 0000 new patients in Europe each year. In 40% of these patients, the cancer will spread to the liver, resulting in liver metastases. This is a serious condition and often fatal. To monitor this condition, patients currently undergo a blood test and liver imaging procedures, which are time consuming, inconvenient and expensive. The COLO-MET project aims to develop an easy, inexpensive test.
Team
- Erasmus MC: Prof. Dr. Jan Ijzermans, Nick van Huizen, Dr. Theo Luider
- Thermo Fisher Scientific: Franck van Veen, Dr. Wilfried Voorhorst, Dr. Gina Tan
- Rotterdam University of Applied Sciences: Margriet Langenberg, Dr. Eddy van der Linden
The COLO-MET project aims to develop an easier, cost-effective and patient-friendly test, involving a urine test combined with a blood test, for early detection of liver metastases, which is associated with bowel cancer. The COLO-MET project will address an important, unmet clinical need for affordable, fast and patient-friendly colorectal carcinoma monitoring. The motivation for this work is to find molecular markers that can aid in simple diagnostics in cancer. The project started with a focus on metastsasis of corectal cancer because of its high social and economic impact.
This project will be performed by a strong public-private consortium. Erasmus MC’s Dr. Theo Luider will act as project leader, and perform retrospective clinical validation of Host Cell Protien (HCP) biomarkers. In collaboration with the world-leader in laboratory equipment Thermo Fisher Scientific, Erasmus MC will optimise the innovative PRM analytical method to achieve high quantification precision and accuracy. Erasmus MC’s Prof. Jan IJzermans will collect patient samples and provide training sessions for clinicians. Throughout the project, students from the Biology and Medical Laboratory research programme of Rotterdam University of Applied Sciences, a programme with a long-standing collaboration with Erasmus MC, will receive on-site training in mass spectrometry techniques, business development and bringing innovations from academia to the market.
The solution can help patients, doctors and the healthcare system by improving monitoring for colorectal carcinoma. The solution offers several specific improvements in healthcare:
- Optimisation of technology for the new biomarker opens the way for accurate determination of other novel biomarkers for colorectal liver metastasis and other indications.
- Use of longitudinal samples may improve biomarker specificity and sensitivity, and will allow the determination of optimal urine collection time for biomarker analysis.
- The PRM method can be optimised for use on HF QE Orbitrap and triple quadrupole available in most clinical laboratories.
Why this is an EIT Health project
By promising to provide earlier warnings of colorectal cancer, this project is in keeping with the EIT Health Focus Area of “Improving Care Pathways”. It also forwards the goal of “Improving Care Pathways”, and the general goals of EIT Health to improve healthcare, because it may help in the identification of other biomarkers.
External partner
- Rotterdam University of Applied Sciences
Members

CLC/InnoStars: Belgium-Netherlands
Partner classification: Education, Research, Hospital / University Hospital
Erasmus University Medical Center Rotterdam (Erasmus MC) is the largest university medical center in The Netherlands with around 16,500 employees, and a top referral hospital for a region of about five million inhabitants. A unique infrastructure bringing multidisciplinary research and healthcare together at one location allows Erasmus MC to excel in fundamental research, fast translation of findings into the clinic including first-in-man testing and clinical implementation, and epidemiological studies.
Technical university medical center: Technology is at the core of the new strategy. Erasmus MC focuses on the use of technology and data science to foster innovation in a variety of fields – from medical instruments and devices, minimally invasive surgery, imaging and image analysis, information technology and smart data technology to artificial intelligence, machine learning and biomedical technology.
Erasmus University Medical Centre Rotterdam
Erasmus University Medical Centre Rotterdam, Dr. Molewaterplein 40, 3015 GD Rotterdam, The Netherlands
Key Activities in Research and Developement
Life Sciences, Social sciences / health economics, Clinical research
Key Activities in Social Innovation
Healthcare provision
Key Activities in Business Creation
Technology Transfer
Key Activities in Education
Medical faculties, Healthcare professional education/training


CLC/InnoStars: Scandinavia
Partner classification: Business
As the Immuno Diagnostic experts within Thermo Fisher Scientific, we develop, manufacture and market complete blood test systems to support clinical diagnosis and monitoring of allergy, asthma and autoimmune diseases.
Thermo Fisher Scientific
Thermo Fisher Scientific, Rapsgatan 7, 754 50 Uppsala, Sweden
Key Activities in Corporate Innovation
Pharma, Diagnostics
Key Activities in Social Innovation
Healthcare provision
Key Activities in Business Creation
Testing & Validation
Key Activities in Education
Medical faculties, Healthcare professional education/training
