Joint pain assessment scoring tool
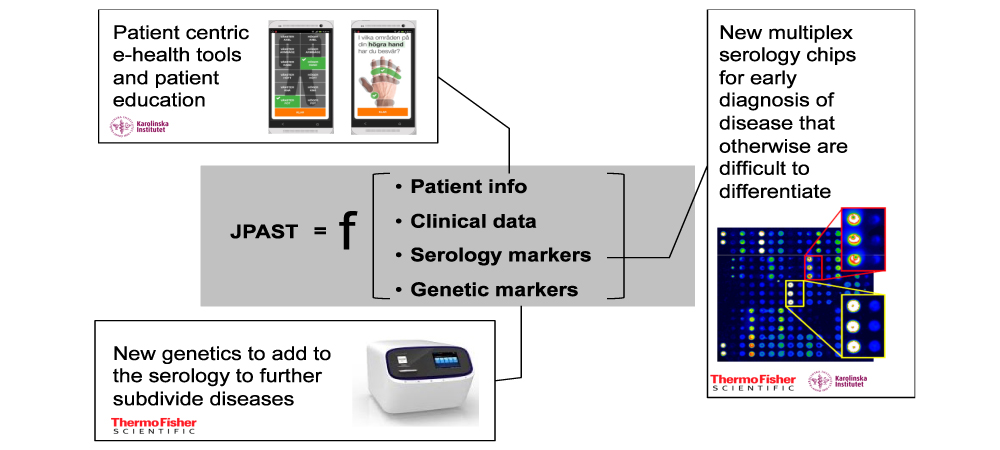
The JPAST project tests and develops innovative new tools that permit earlier diagnosis of Rheumatic and Musculoskeletal Diseases (RMDs) – with the ultimate goal of bringing those tools to market. Innovations introduced by the project include diagnostic and e-health tools and a new risk score: JPAST (Joint Pain Assessment Scoring Tool).
Origins
RMDs affect up to 5% of the population in Europe and the number of those afflicted is rising, due to our ageing society. The correct diagnosis of RMDs is often delayed, causing disability and reduced quality of life for individuals, as well as high costs for society. Through earlier interception of the disease, JPAST allows accurate stratification of patients, engagement and education of citizens and healthcare providers, and the implementation of new diagnostic strategies.
Team
The EIT Health Innovation Project JPAST is jointly led by Karolinska Institutet and Thermo Fisher, which provides both deep scientific knowledge and industrial and business development experience. Validation partners include clinicians and leading scientists/clinicians at Leiden University, University of Birmingham, Uppsala University and Friedrich-Alexander-Universität Erlangen-Nürnberg.
The project
The aim of JPAST is to accelerate the market introduction of tools that combine use of biomarkers, genetics and e-health to drastically improve early diagnosis of RMDs.
The project includes four main activity blocks:
- Identifying individuals with joint pain through a web-based self-assessment tool and involving them in the project to allow structured patient work up and early education/awareness.
- Developing and validating JPAST and the underlying products and services in clinical settings in several European countries.
- Implementing JPAST in selected laboratories to permit analysis of samples sent in from throughout the region.
- Using Big Data to achieve faster therapy selection and to identify patients for clinical trials in very early stages of the disease.
Impact
JPAST promises to facilitate earlier diagnosis of RMDs. This will mean a better quality of life for patients, who will be able to take more control over their own health and see how different lifestyle choices and treatments could affect the disease’s progression. Society benefits because the costs of prevention are lower than the costs of treatment, and because patients who can better manage their disease will remain productive longer.
Why this is an EIT Health project
This project addresses the EIT Health core goal of promoting active ageing. It also falls into several EIT Health Focus Areas:
- “Care Pathways”, because early diagnosis allows for preventive measures that can improve the course of RMD.
- “Behavioural Change”, because it encourages patients to manage their RMD.
- “Real World Data”, because it employs Big Data.
Members

CLC/InnoStars: Germany
Partner classification: Education, Research, Hospital / University Hospital
Founded in 1743, FAU has a rich history. It is a strong research university with an international perspective and one of the largest universities in Germany, with 39,780 students, 265 degree programmes and 4,000 academic staff. At Universitätsklinikum Erlangen (the university hospital), 7.400 employees promote health and cure disease. With up-to-date equipment and science-based diagnostic and therapeutic procedures, the 24 departments of the FAU, 25 clinical departments, 19 institutes, and 21 independent departments at Universitätsklinikum Erlangen comprehend every field of modern medicine.
Friedrich-Alexander-Universität Erlangen-Nürnberg
Friedrich-Alexander-Universität Erlangen-Nürnberg, Schloßplatz 4, 91054 Erlangen, Germany
Key Activities in Research and Developement
Biomedical engineering, Life Sciences, Social sciences / health economics
Key Activities in Social Innovation
Healthcare provision
Key Activities in Business Creation
Incubation, Technology Transfer
Key Activities in Education
Entrepreneurship training, Technical faculties, Medical faculties


CLC/InnoStars: Scandinavia
Partner classification: Education, Research
Karolinska Institutet is one of the world's leading medical Universities. Our mission is to contribute to the improvement of human health through research and education. Our research covers the entire medical field, from basic molecular biological research, to clinical epidemiology and nursing science. Since 1901 the Nobel Assembly at Karolinska Institutet has selected the Nobel laureates in Physiology or Medicine. With our close relationship to the clinical world, a well-established infrastructure, a unique innovation system and financial stability, Karolinska Institutet has excellent prerequisites for sustaining high-quality research and education.
Key Activities in Corporate Innovation
Pharma, Med Tech, Diagnostics, Imaging, Nutrition
Key Activities in Social Innovation
Healthcare provision
Key Activities in Business Creation
Incubation, Technology Transfer, Business coaching, Testing & Validation
Key Activities in Education
Entrepreneurship training, Medical faculties, Healthcare professional education/training


Partner classification: Education, Research, Hospital / University Hospital
The mission of the LUMC is ‘to be an innovator improving both healthcare and population health’. LUMC is a university medical center for research, education and patient care with a high quality profile and a strong scientific orientation. It has a unique research practice, ranging from pure fundamental medical research to applied clinical research. LUMC wants to perform ground-breaking innovation. Researchers, educators and healthcare providers at LUMC work together around 3 societal outreach topics, Oncology, Regenerative Medicine and Population Health. Furthermore 10 themes for innovation are defined Academic Pharma, Neuro Science, Cancer, (auto)Immunity, Cell, Tissue & organ, Cardio-Vascular, Genetics, infection, Life course and prevention and Lifestyle. https://strategie.lumc.nl/en/
Leids Universitair Medisch Centrum
Leids Universitair Medisch Centrum, Albinusdreef 2, 2333 ZA Leiden, Netherlands
Key Activities in Research and Developement
Life Sciences, Clinical research
Key Activities in Social Innovation
Healthcare provision
Key Activities in Business Creation
Incubation, Technology Transfer


CLC/InnoStars: Scandinavia
Partner classification: Business
As the Immuno Diagnostic experts within Thermo Fisher Scientific, we develop, manufacture and market complete blood test systems to support clinical diagnosis and monitoring of allergy, asthma and autoimmune diseases.
Thermo Fisher Scientific
Thermo Fisher Scientific, Rapsgatan 7, 754 50 Uppsala, Sweden
Key Activities in Corporate Innovation
Pharma, Diagnostics
Key Activities in Social Innovation
Healthcare provision
Key Activities in Business Creation
Testing & Validation
Key Activities in Education
Medical faculties, Healthcare professional education/training


CLC/InnoStars: Scandinavia
Partner classification: Education, Research, Tech Transfer, Clusters, Other NGOs
Partner type: Associate Partner
The first university established in the Nordic countries founded in 1477, is an international research university. Uppsala University is ranked #61 in the Academic Ranking of World Universities 2014. World-class research and high at UU benefit society and business on a global level. The University is characterized by diversity and breadth, with international frontline research at nine faculties including 40,000 students, 1,800 teachers and researchers whereof about 670 professors. Comprehensive peer reviews and university rankings consistently show that research at UU is of the highest international standard. Research, education and innovation serve as guiding concepts in connection with cooperation with the business community and society at large
Key Activities in Corporate Innovation
Pharma, Med Tech, ICT, Diagnostics, Imaging
Key Activities in Social Innovation
Healthcare provision
Key Activities in Business Creation
Incubation, Technology Transfer, Business coaching, Testing & Validation
Key Activities in Education
Business Schools, Entrepreneurship training, Technical faculties, Medical faculties, Healthcare professional education/training
