Revolutionising drug development by making toxicity testing more human
Developing a ready-to-use toxicity analysis based on human cells
Testing new drugs for toxicity is an essential part of the drug development process. Toxicity can have major consequences for people, from skin irritation to cancer.1
Screening drug candidates for toxicity early in the development process is not only important to avoid endangering public health, it can also reduce the need to withdraw drugs further along in the process, such as at clinical trials, which is both costly and wasteful. As many as 30% of drug candidates must be withdrawn at clinical testing or later due to toxicity.2
Conventionally, drug screening has relied on live animals or animal cells and tissues. However, animal models do not always accurately predict human responses because toxicity is species-dependent and the underlying biochemistry is often complex and poorly understood.2
Although technology-based toxicity screenings of humans are available, the cost, workload and expertise required make them too demanding for routine use. A tool that enables testing in human tissue could improve the accuracy of predicting human responses to drugs before they go to clinical trial.
Reproducing human cells quickly and cost-effectively
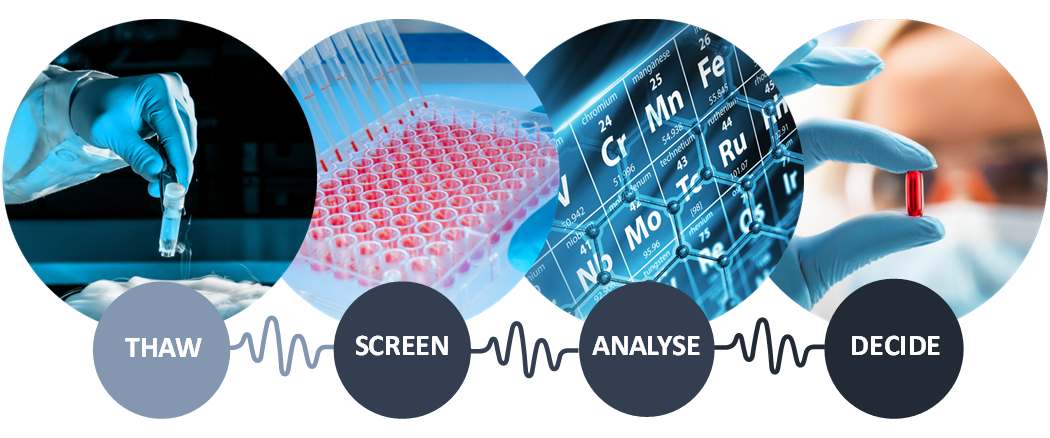
A ready-to-use toxicity assay (R2U-Tox-Assay) is a test for toxicity using plates that provide a biologically active environment. Within this system, maturated heart and nerve cell models can be quickly and cost-effectively produced from human-induced pluripotent stem cells, or hiPSCs.
The ready-to-use plates undergo cryopreservation, or freezing, and are shipped direct to customers. Freezing reduces any variability during travel. This strict, ready-to-use approach means the end customer can perform screenings with minimal effort. The product is also designed to integrate easily in conventional drug screening workflows of pharmaceutical companies.
EIT Health is helping bring human diversity to the petri dish to improve drug development
EIT Health’s support has a clear focus: to enable inventors to transform innovative ideas in a proof-of-concept state into a market-ready product or service. Innovations like R2U Tox-Assay will not only improve the lives of patients but also help clinicians detect human toxicity much earlier.
Thanks to the EIT Health consortium, the various aspects of the project were able to fuse together. These included the timely and cost-effective expansion of hiPSCs, robust differentiation of high-quality tissue models, and processes and tools to deliver the screening system to customers.
The team behind the screening tool combined translational and market-driven R&D of ready-to-use products. One of the partners, Janssen Pharmaceutical NV, is also interested in using the Tox-Assay as a valuable R&D tool for future drug development.
References
- Parasuraman S. Toxicological screening. J Pharmacol Pharmacother 2011; 2(2): 75–79.
- Hvastkovs EG and Rusling JF. Modern approaches to chemical toxicity screening. Curr Opin Electrochem 2017; 3(1): 18–22.
Partners
Partner classification: Business
Partner type: Linked/Affiliated Party
Janssen Pharmaceutica NV
Janssen Pharmaceutica NV, Turnhoutseweg 30, 2340 Beerse, Belgium

CLC/InnoStars: Spain
Partner classification: Research
Partner type: Linked/Affiliated Party
The Institute of Bioengineering of Catalonia (IBEC) conducts excellent interdisciplinary research between engineering and life sciences in order to generate new knowledge and join fields such as nanomedicine, biophysics, biotechnology, tissue engineering and the applications of health information technology. IBEC’s specialization areas are 3D Bioprinting, organ on a chip, microfluidics & lab on a chip, iPSCs for disease modeling and drug testing, HR microscopy at micro/nanoscale, advanced signal & data processing, nanorobotics, fabrication of biomaterials and characterization, biofunctionalization, engineering of bioactive molecules, biosensors, micro/nano electronics, neuroengineering and big data.
Institute of Bioengineering of Catalonia (IBEC)
Institute of Bioengineering of Catalonia (IBEC), Carrer de Baldiri Reixac, 10-12, 08028 Barcelona, España



Cryo and Stem Cell Technology | Head of Department | Fraunhofer Institute for Biomedical Engineering IBMT
Contact
Neuroscience Discovery | Senior Scientist | Janssen Pharmaceutica NV
Contact
Pluripotency for organ regeneration | Group Leader, ICREA Research Professor | Institute for Bioengineering of Catalonia IBEC
Contact