AI powers the fight in clinical research to prevent heart failure
RealWorld4Clinic creates device connected by artificial intelligence (AI) to enhance patient care.
Heart failure is responsible for five per cent of emergency hospital stays and two per cent of national health expenditure within Europe1 – figures that are unacceptably high.
The difficulty is that developing a new cardiac drug from idea to market costs up to €1.5 billion and takes approximately 15 years – and a high proportion of new drugs are unsuccessful. This lack of drugs to address heart failure threatens citizens’ lives, generates significant costs and increases unrealistic expectations from patients, healthcare providers and payers.
Meanwhile it’s widely accepted that real-world data is vital for diagnosing heart failure early. Yet clinical research and heart failure management still buy in data, limiting the predictive power of clinical trials.
Inspired by consumer wearable devices, RealWorld4Clinic, set about creating a wearable that could collect data suitable for drug approval trials and guide interventions in heart failure management.
The coin-sized device transforming heart research
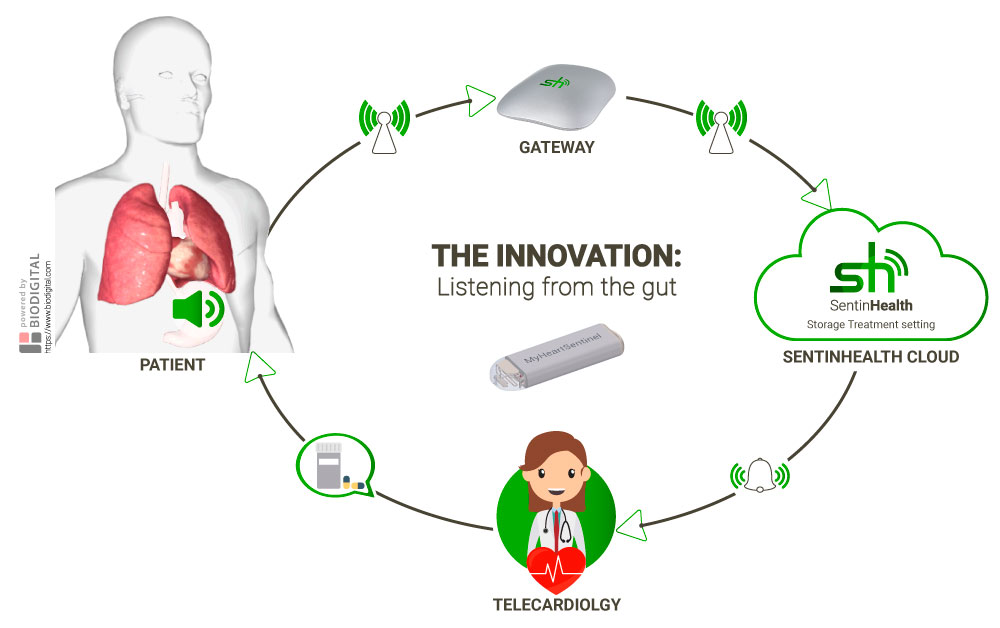
RealWorld4Clinic has created MyHeartSentinel, a medical device that dovetails greater patient data, clinical research and clinical care.
Anchored to the fundus of the stomach in immediate proximity to the heart and the lung, the device gathers continuous real-world data about the state of the patient’s cardio-respiratory health. AI then makes sense of the data to aid drug development and telecardiology.
RealWorld4Clinic aims to enhance the quality of early decision-making by pharmaceutical companies on clinical development programmes. It’s estimated that this device could help to improve the predictive ability of clinical trials by 50%.
Big ambitions to move from reactive to proactive heart health
MyHeartSentinel is just the beginning. Supported by EIT Health, the RealWorld4Clinic consortium is committed to transforming the standard of care from reactive to proactive heart health management. By diagnosing heart failure aggravation from 30 days before an emergency event occurs, RealWorld4Clinic will halve the number of days patients spend in a hospital, while reducing hospital costs.
In addition to co-creating the wearable MyHeartSentinel product, EIT Health will help resolve the ethical, legal and social issues involved with buying and using AI to maximise heart health data.
Looking forward, EIT Health will help RealWorld4Clinic navigate the process to gain the certification mark application for MyHeartSentinel and prepare to scale up the business for drug development for the outpatient heart care market.
“All of us share the aim to break down innovation barriers and to quickly provide thoroughly-validated solutions to the people who need them most.”
Professor Dr. Freimut Schliess, Director Science & Innovation, Profil GmbH
External partners
- aQua Institute
- CHU Rennes
- LINQ
- SurgiQual Institute
- Zana Technologies
- PrYv
Linked Third Parties
- CHUGA
- Profil Mainz
Notes
[1] The Heart Failure Policy Network: A policy brief on heart failure in Europe, March 2015: http://www.healthpolicypartnership.com/wp-content/uploads/HFPN-Policy-Backgrounder-March2015-FINAL-EXTERNAL1.pdf (accessed March 2020)
Members

CLC/InnoStars: France
Partner classification: Research, Tech Transfer, Clusters, Other NGOs
Founded in 1964, the French National Institute of Health and Medical Research (Inserm) is a public scientific and technological institute which operates under the joint authority of the French Ministry of Health and French Ministry of Research. As the only French public research institute to focus entirely on human health, in 2008 Inserm took on the responsibility for the strategic, scientific and operational coordination of biomedical research. This key role as coordinator comes naturally to Inserm thanks to the scientific quality of its teams and its ability to conduct translational research, from the laboratory to the patient’s bed. Inserm plays a leading role in creating the European Research Area and boosts its standing abroad through close partnerships (teams and partner laboratories abroad). Inserm is active in many areas, such as Technologies for Health, Public Health (Cohorts, Healthcare systems), Infectious and chronic disease, Neurosciences, Cancer, and Genomics.
INSERM - French National Institute of Health and Medical Research
INSERM - French National Institute of Health and Medical Research, 101 Rue de Tolbiac, 75013 Paris, France
Key Activities in Corporate Innovation
Pharma, Med Tech, ICT, Diagnostics, Imaging, Nutrition
Key Activities in Social Innovation
Healthcare provision
Key Activities in Business Creation
Incubation, Technology Transfer
Key Activities in Education
Medical faculties, Healthcare professional education/training

CLC/InnoStars: Germany
Partner classification: Education, Research, Tech Transfer, Clusters, Other NGOs, Hospital / University Hospital
RWTH Aachen University and its hospital (focus on patient-oriented medicine & nursing care) provide leading research, innovation and education within the core themes of EIT Health. Our industry-need-driven competence centres foster entrepreneurship
RWTH Aachen University
RWTH Aachen University, Templergraben 55, 52062 Aachen, Germany
Key Activities in Research and Developement
Biomedical engineering, Life Sciences, Social sciences / health economics, Clinical research
Key Activities in Business Creation
Incubation, Technology Transfer, Testing & Validation
Key Activities in Education
Entrepreneurship training, Technical faculties, Medical faculties, Healthcare professional education/training

CLC/InnoStars: France
Partner classification: Education, Research, Tech Transfer, Clusters, Other NGOs
The UGA in Grenoble is a leading University of Science Technology and Health. Within 80 multidisciplinary laboratories, the UGA is developing outstanding research at national and international level. The UGA offers a wide range of training courses, from bachelor to doctorate, in close connection with the socio-professional environment to promote the integration of its students.
Université Grenoble Alpes (UGA)
621 Avenue Centrale, 38400 Saint-Martin-d'Hères
Key Activities in Research and Developement
Biomedical engineering, Life science, Social sciences /health economics
Key Activities in Corporate Innovation
Pharma, Med Tech, ICT, Diagnostics, Imaging, Nutricion
Key Activities in Business Creation
Incubation, Technology Transfer, Business coaching
Key Activities in Education
Entrepreneurship training, Technical faculties, Medical faculties, Healthcare professional education/training


CLC/InnoStars: France
Partner classification: Education, Research
Partner type: Associate Partner
Grenoble Ecole de Management (GEM) is more than just a business school. GEM represents an open-ended laboratory through which 8,000 students and 500 employees learn and work every day to solve complex problems and overcome major challenges for business and society. Founded in Grenoble, a city of science and technology, our school has greatly developed its expertise in the management of technology and innovation—By developing its own educational model, GEM has become a center for experimentation, study and creation. Our research teams generate knowledge that fuels new content for our training programs and offers companies a better understanding of their challenges in order to help them define solutions which meet their needs. All of these initiatives underline our ambition to become an influential school whose actions, thoughts and projects help improve the well-being of society—an ambitious goal to truly become a 'Business Lab for Society'.
Grenoble Ecole de Management (GEM)
12 Rue Pierre Sémard, 38000 Grenoble
Key Activities in Research and Developement
Other research, Social sciences /health economics
Key Activities in Corporate Innovation
Consumer products, Nutrition
Key Activities in Business Creation
Testing & validation
Key Activities in Education
Business schools


CLC/InnoStars: France
Partner classification: Research, Tech Transfer, Clusters, Other NGOs
MADoPA is a Living Lab specialising in co-creation, experimentation and evaluation of technologies and services for the elderly. MADoPA is a non profit association created in 2009, financially independent and involved in multiple European, national and regional projects.
MADoPA
MADoPA, 2 Rue Gustave Eiffel, 10430 Rosières-prés-Troyes, France
