Mobile Autonomy for Children in end-stage Heart failure
The new EXCOR® Active driving unit, developed by the MACH project, means that children who are awaiting heart transplants will no longer be forced to live in a hospital bed, connected to stationary machinery for weeks or months. The MACH project was established to achieve CE certification and market entry of EXCOR®. The project was a success as both of these goals have been achieved as of 2020.
Origins
Children in end-stage heart failure awaiting heart transplantation face greater difficulty with a shortage in donor organs than adults. The number of children waiting on the heart transplant list is growing but donation rates are not. Children and adults awaiting transplant, often need Ventricular Assist Devices, or artificial hearts, to bridge the waiting time for a new heart. The EXCOR® is the only mobile version of such a device for children.
Team
The EIT Health project MACH is a joint action by the RWTH Aachen University and Newcastle University. Both members are supported by the University Hospital RWTH Aachen and the Newcastle Upon Tyne Hospital. As an external business partner, the company Berlin Heart GmbH, and their system for mechanical circulatory support, EXCOR® Paediatric, is in the centre of this project.
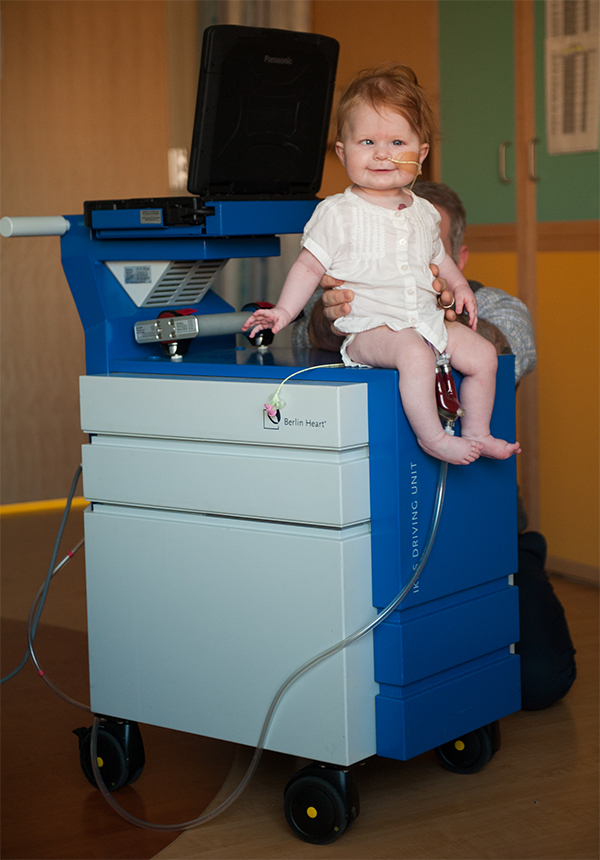
(Photos on this page with kind permission from Joni Schrantz, Photographer.)
The project
The main goal of EIT Health MACH is CE certification and market entry of the new EXCOR® Active driving unit. Certification and market entry have been achieved thanks to this project, and the new product is expected to revolutionise how we perceive mechanical circulatory support therapy in the paediatric population. While the first year of the project created the necessary structures and networks, the second year of the project was mainly devoted to testing and education.
Testing has picked up speed. In a parallel setting of both human and animal blood testing, the effects of the alternative pressure curve induced by the new driver unit were analysed with regard to haemocompatibility, to shear stress and to activation of the coagulation cascade.
The educational work involved initiation of a platform for health care professionals and private persons to be trained on paediatric mechanical circulatory support. One of the main outputs of the final year of the EIT Health MACH action will be the release of the educational platform, which offers training for health care professionals, patients, their families and caregivers.
Impact
The new driving unit will give children awaiting transplants a certain degree of freedom and a chance for a better quality of life. It will also improve quality of life for their families, who won’t have to constantly visit a hospital. Development of a portable driver will also reduce treatment costs, create new jobs and increase life quality. The device will also reduce pressure on the limited supply of hospital beds.
Why this is an EIT Health project
EIT Health is eager to support a project of such importance to the growing number of children in end-stage heart failure. The project is aligned with the EIT Health Focus Areas of “Bringing Care Home”, because it makes it possible for children to leave the hospital, and “Care Pathways” because it improves care of a specific ailment.
Members

CLC/InnoStars: Ireland-UK
Partner classification: Education, Research
Partner type: Associate Partner
Newcastle University has always focused on academic excellence and the impact of their academic work. They seek to address some of the key global societal issues through research in 3 areas: ageing; social renewal; sustainability. Newcastle University collaborates with a range of partners and strategic initiatives. These partnerships help to extend the University’s influence and reinforce their ties with the city, the region and beyond.
Newcastle University
Newcastle University, Newcastle Biomedical Research Building, Newcastle upon Tyne NE4 5PL, UK
Key Activities in Corporate Innovation
Med Tech, ICT
Key Activities in Business Creation
Finance & Investment, Business coaching
Key Activities in Education
Business Schools, Entrepreneurship training, Technical faculties, Medical faculties

CLC/InnoStars: Germany
Partner classification: Education, Research, Tech Transfer, Clusters, Other NGOs, Hospital / University Hospital
RWTH Aachen University and its hospital (focus on patient-oriented medicine & nursing care) provide leading research, innovation and education within the core themes of EIT Health. Our industry-need-driven competence centres foster entrepreneurship
RWTH Aachen University
RWTH Aachen University, Templergraben 55, 52062 Aachen, Germany
Key Activities in Research and Developement
Biomedical engineering, Life Sciences, Social sciences / health economics, Clinical research
Key Activities in Business Creation
Incubation, Technology Transfer, Testing & Validation
Key Activities in Education
Entrepreneurship training, Technical faculties, Medical faculties, Healthcare professional education/training