A clinical translation of tissue engineering to treat delayed or non-union fractures
OsteoLife develops an implant for poorly healing fractures, a common problem among older patients. EIT Health is supporting preparation of human testing of this innovation, which uses cells that are derived from bone tissue and grown on a calcium phosphate scaffold to treat fractures of long bones, especially the tibia, or shin bone.
Origins
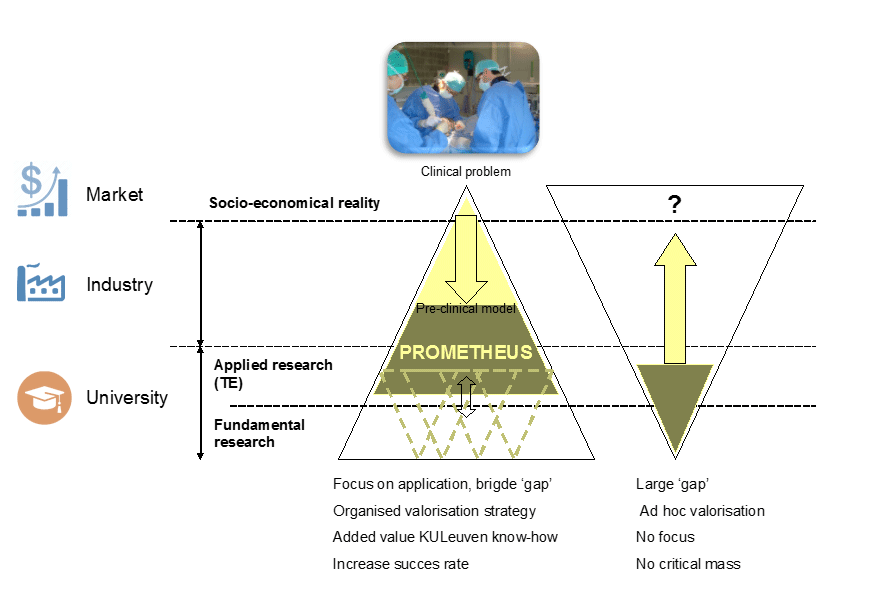
TiGeniX, a biotech spin-off of KU Leuven, developed ChondroCelect® – which in 2009 became the first advanced therapeutic medicinal product (ATMP) to gain EU marketing approval. Prometheus, the division of Skeletal Tissue Engineering of KU Leuven, is now leveraging this experience by creating a new spin-off, producing novel ATMPs to treat bone and cartilage injuries for non-unions (fractures that won’t heal) and osteochondral repair.
Team
The project lead is Prof. Frank Luyten, Director of Prometheus and Co-founder of TiGenix. He brings extensive experience, particularly in navigating the complex regulatory landscape and in obtaining early stage investment. Dr. Hermann Oppermann of Genera Research Ltd. contributes extensive knowledge and experience in creating novel, recombinant Bone Morphogenetic Proteins.
The project
Europe is facing an increasing need for a cost-effective solution to restore damaged skeletal tissue in an ageing population, in order to ensure mobility and independence. The OsteoLife consortium has developed a novel solution: a tissue-engineered implant consisting of periosteum derived (stem) cells seeded on a calcium phosphate scaffold coated with growth factor (Bone Morphogenetic Protein).
EIT Health funding will contribute to finalising pre-clinical testing of the implant, as well as updating the necessary Ethical Committee and Regulatory Authority dossiers, in order to move into clinical testing in humans.
The intellectual property generated from this work will contribute to the creation of a spin-off company, and will form the foundation for attracting the capital investment required for the further clinical development and scale-up of GMP manufacturing processes, in order to make the implant available to the target population.
Impact
OsteoLife promises to help patients whose quality of life would suffer greatly due to non-healing fractures that require recurrent surgeries – and in a worst-case, amputation. Payers benefit as the solution can lead to a reduction in hospital costs by up to 50% (particularly in ageing patients with osteoporosis). And society’s economic burden will also be reduced by enabling patients to return to productive activity.
Why this is an EIT Health project
Osteo-Life contributes to the EIT Health challenge of supporting active ageing by commercialising a new, regenerative-medicine-based therapy designed to overcome functional loss in mobility that particularly afflicts older adults. It is aligned with the EIT Health Focus Area of “Health Continuum Care Pathways” because it seeks evidence-based implementation of an innovative health delivery solution.
Partners

CLC/InnoStars: Belgium-Netherlands
Partner classification: Education, Research
Partner type: Associate Partner
KU Leuven (together with University Hospitals Leuven) is a research-intensive, internationally oriented university that carries out excellence-driven research in health and care and is dedicated to build bridges between science, society and industry.


