Functional signalling pathway activity complementing DNA Mutation analysis for precision medicine
PACMAN will test how well the OncoSignal solution increases the effectiveness of new, personalised cancer therapy. Significant progress has been made with targeted drugs that block tumour-driving molecular pathways, but it is still difficult to predict how patients will respond. OncoSignal was designed to improve selection of optimal personalised therapy for individual patients.
Origins
In the Moscato-01 trial performed by Gustave Roussy Cancer Center, 843 hard-to-treat cancer patients were characterised based on DNA mutation analysis. An outcome benefit was observed for only 7% of the patients. Through quantitative assessment of aberrant functional activity of signalling pathways that drive cancer growth and progression, OncoSignal could improve this number by identifying more patients who benefit from targeted therapy.
Team
Gustave Roussy Cancer Campus in France is the number one cancer centre in Europe, running 428 clinical trials in 2017. Dr Christophe Massard MD, the PI of the Gustave Roussy MOSCATO study is responsible for all clinical aspects related to this project. MPDx is a new business within Philips that is responsible for development and marketing of OncoSignal molecular pathway tests.
The project
The main objective of this project is to increase the number of patients who can be assigned to clinical trials with targeted drugs and to increase the number of patients for which response/resistance to targeted therapy is correctly predicted. Using tissues from the MOSCATO-01 trial, OncoSignal pathway analysis will be performed on tumour tissues without targetable mutation and treated targetable tumours, to validate that OncoSignal can identify more patients who will benefit from targeted therapy. Subsequently, further validation will be done by employing OncoSignal in prospectively running clinical studies at Gustave Roussy.
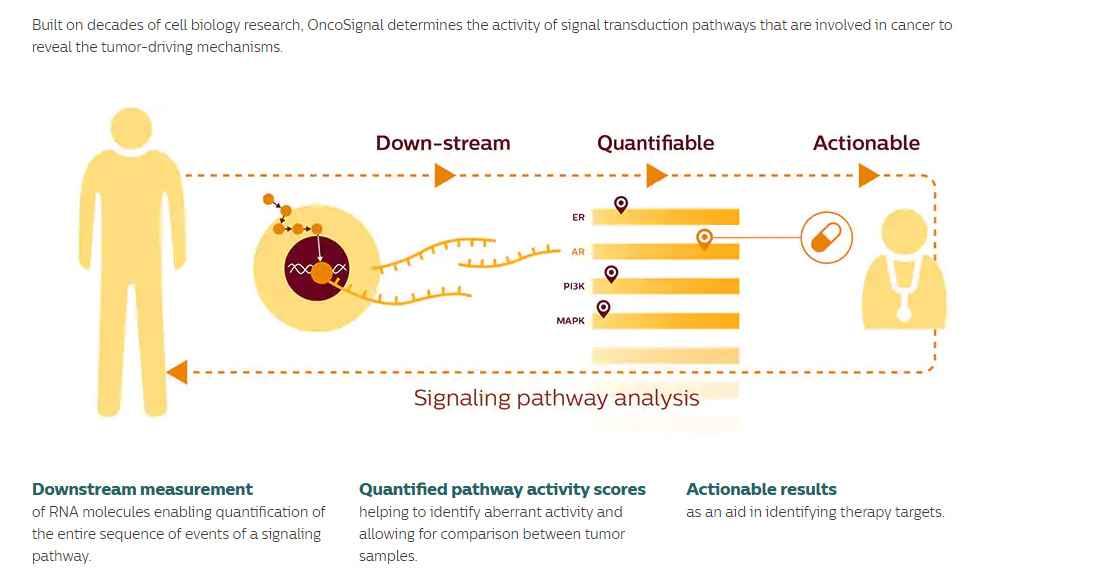
For 200 patients, OncoSignal analysis will be performed complementary to DNA mutation analysis. Patients with identifiable targets based on DNA mutations are assigned to defined clinical trials. OncoSignal pathway activity information can be consulted when patients develop resistance against chosen therapy. For patients that cannot be assigned to a clinical trial based on DNA mutation analysis, new options may be opened to refer to a clinical trial based on OncoSignal pathway activity results.
EIT Health support to PACMAN enables clinical validation of the OncoSignal technology and allows further development of regulatory, reimbursement and marketing aspects for the pan-cancer application.
Impact
OncoSignal will contribute to disruption of cancer care by moving from treatment based on cancer type to tumour biology. Assessment of the functional molecular phenotype will provide improved therapy response prediction compared to DNA genotyping analysis alone. The project promises to:
- improve clinical outcomes, especially for hard-to-treat cancers;
- reduce unnecessary side effects from ineffective therapies;
- save costs due to more effective (personalised) therapies.
Why this is an EIT Health project
By validating a solution that would allow more patients to be treated with promising new personalised cancer therapies, PACMAN is in line with the EIT Health Focus Area of “Improving Care Pathways”. In particular, the testing involved in PACMAN paves the way for evidence-based improvements in healthcare.
Partners

CLC/InnoStars: Belgium-Netherlands
Partner classification: Business
Partner type: Associate Partner
Philips Electronics Nederland B.V.
Philips Electronics Nederland B.V., Boschdijk 525, 5622 Eindhoven, Netherlands
Key Activities in Corporate Innovation
Med Tech, ICT






CLC/InnoStars: France
Partner classification: Education, Research, Hospital / University Hospital
Partner type: Associate Partner
Gustave Roussy is the premier European Cancer Centre uniting patient care, research, and teaching that places innovation at the heart of a human, scientific, and technological revolution in the fight against cancer.
Gustave Roussy Cancer Campus Grand Paris
Gustave Roussy Cancer Campus Grand Paris, 114 Rue Edouard Vaillant, 94800 Villejuif, France
Key Activities in Research and Developement
Other research, Life science, Clinical research
Key Activities in Social Innovation
Healthcare provision
Key Activities in Business Creation
Testing & validation
Key Activities in Education
Medical faculties, Healthcare professional education/training


