4th November 2021
The company mjn-neuro, backed by EIT Health, a European community of innovators, launched its device in Spain last year, and is looking to expand onto international markets.
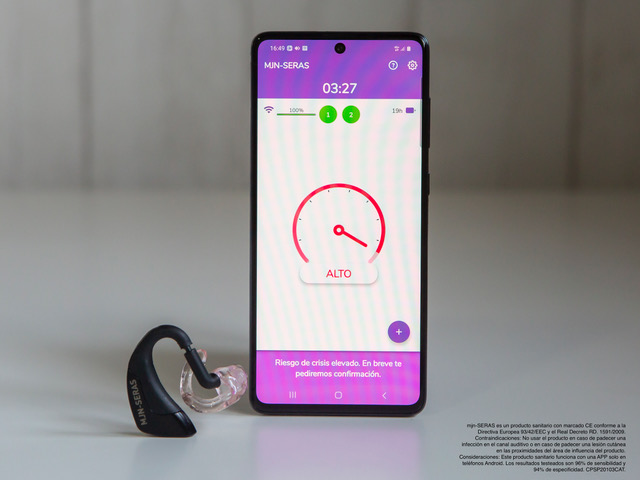
The device warns people with epilepsy of a potential seizure minutes before it takes place, thus preventing accidents and alerting the people around them.
Girona-based company mjn-neuro is looking to expand its presence on the international market, using the more than 1 million euros it raised in a funding round that kicked off last May on the Capital Cell platform. This financial boost is accompanied by agreements the company has reached on markets such as the United Kingdom, the Netherlands, and Germany, the country where it has been supported by EIT Health through the Bridgehead 2020 programme.
With this funding round, the company has reached 100% of its crowdfunding goal in less than five months, and hopes to reach 120% in the coming months. In this period, the company’s value has tripled and currently stands at 12 million euros, following participation from US and Spanish investors. What is more, the round has been extended to allow for the entry of a major family office from Denmark.
This is how the company aims to sell its mjn-SERAS device in Europe, with a view to making the most of market traction and launching a new funding round next year. To date, an agreement has been closed with expert provider Epilepsy Alarms for the distribution of the solution in the UK, and a preliminary agreement has been reached with another distributor in the Netherlands. Additionally, mjn-neuro has made its first contact with the German market through Medical Valley, in a key move for its expansion strategy, thanks to EIT Health’s Bridgehead 2020 programme.
EIT Health’s Bridgehead: a bridge to international markets
Support from EIT Health – the biggest community of innovators in Europe, backed by the EU – has been crucial for the company to be able to establish contact with big-name partners on the worldwide medical scene. In 2020, mjn-neuro was selected to participate in the Bridgehead Europe programme: an initiative that shines the spotlight on European startups to put them in touch with business incubators and startup accelerators in the EIT Health network on the European continent, with the ultimate aim of facilitating their expansion onto other markets in the quickest, most emphatic way possible.
This building of bridges between different markets has enabled mjn-neuro to access relevant partners, establish reimbursement systems, and define its value proposition and competitive edge over the competition. In addition, the programme has brought together distributors specialised in epilepsy and opinion leaders in this area of research. In 2021 mjn-neuro will take part once again in the Bridgehead programme and aims to replicate the process started with Germany during the 2020 edition, but this time on the French market.
‘It is always a pleasure to support projects that help patients to improve their quality of life. Here at EIT Health Spain, we promote initiatives to ensure these innovative ideas get on the market and even go international, as is the case here. We will definitely continue to work side by side with mjn-neuro to make sure the solution reaches as many people as possible, in Spain and beyond’, explains Cristina Bescos, managing director of EIT Health Spain.
‘Following our success in Spain, we want to keep working to ensure our product reaches everyone who needs it and improve their quality of life’, declares the CEO of mjn-neuro, David Blánquez. ‘We are grateful for EIT Health’s support in helping us reach new markets through the Bridgehead Europe programme, through which we have established contact with other incubators and accelerators on the European scene. They have become crucial partners in our efforts to expand mjn-neuro beyond our borders’.
The creator of this innovative device has a child with epilepsy
David Blánquez, CEO of mjn-neuro and father of Marina, who has been living with epilepsy for more than 17 years, decided to use his talent and knowledge as an IT engineer to come up with a device that would warn his daughter and other people with epilepsy of a seizure before it happens, thus giving them time to take safety measures to minimise the consequences.
To do so, he teamed up with Salva Gutiérrez and Xavier Raurich, and they created mjn-SERAS: an earpiece, similar to an external hearing aid, that uses sensors to monitor brain activity through the ear canal in real time. Personalised artificial intelligence algorithms then analyse this information and determine the risk of a seizure. The device, connected to a smartphone through an app, sends an alert to the patient and their chosen contacts if the risk of seizure is high.
The company mjn-neuro is backed by the European Institute of Innovation and Technology (EIT Health) and the European Commission on various projects. In 2018, the project received a grant of 1.8 million euros from the Horizon2020 SME Instrument programme, led by the European Commission, as well as 550,000 euros from the Spanish government’s ENISA organisation and ‘Retos-Colaboración’ (‘Challenges-Collaboration’) scheme. What is more, this year, CDTI awarded a grant of 2.1 million euros, some of which went to the startup so that it could work on other innovations in the field of neurology, in order to provide solutions to various neurological diseases. Since it was founded, mjn-neuro has received a total of 4 million euros from public grants, loans and private investment.
Health experts make recommendations on EHDS implementation

Discover our new Think Tank report.
EIT Health-supported ABLE Human Motion receives CE mark

Learn how EIT Health has supported their journey.

